April 16, 2024
The U.S. Food and Drug Administration cleared a first-of-its-kind AI application for hemorrhage triage of combat casualties. Developed by the U.S. Army Medical Research and Development Command (MRDC)’s Biotechnology High Performance Computing Software Applications Institute (BHSAI) and HJF Research Scientists and Software Developers, the APPRAISE-Hemorrhage Risk Index (HRI) provides medics the means to automatically screen Service members for hemorrhage risk after a physically traumatic event and stratify casualties who need immediate attention and emergency evacuation.
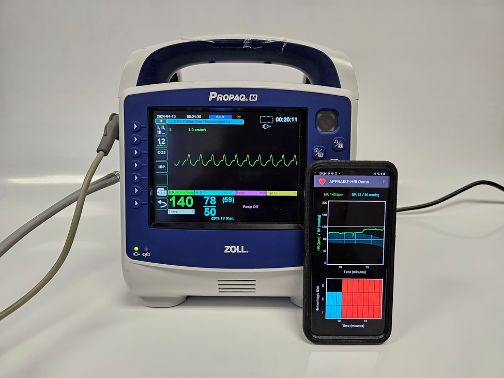
Hemorrhage is the leading cause of preventable death on the battlefield, where more than 90% of combat casualties die before ever reaching a medical treatment facility. To address the challenge of identifying trauma casualties at risk for uncontrolled bleeding at the point of injury, the APPRAISE-HRI application can stratify the risk of hemorrhage within 10 minutes, greatly assisting medics in triaging casualties in prolonged field care scenarios with limited resources in time to improve their chances of survival.
The APPRAISE-HRI consists of a commercial vital-sign monitor and an AI app running on an Android smartphone. The monitor continuously collects routine heart rate and blood pressure data from the casualty and wirelessly transmits the data to the smartphone, where the AI app analyzes patterns in the vital signs and provides a hemorrhage risk score. The APPRAISE-HRI offers the unique capability to automatically integrate and extract information from standard vital signs in a systematic and reproducible manner, to improve situational awareness and help medics identify the most severely injured casualties. The device’s risk score is easy to interpret and the use of standard vital signs will minimize training and facilitate dissemination.
To train the AI algorithm, we conducted three clinical studies to collect real-world vital-sign data from about 2,000 civilian trauma casualties either during ground- and air-ambulance transport from the point of injury to a receiving hospital or in the Emergency Department. To obtain the FDA 510(k) clearance, we blindly and independently assessed the AI algorithm using vital-sign data collected from an additional 6,000 trauma patients at nine different sites. Based on the results of the independent assessment, the FDA concluded that the APPRAISE-HRI application is “a useful tool to aid in discriminating hemorrhage risk in the trauma population.”

Photo: Dr. Stephen Dalal (left) and Betty Crosby (far right) recognizing the effort of HJF personnel on the APPRAISE-HRI team above: Left to right: Maria Kuhrmann, Francisco Vital-Lopez, Valmik Desai, Jeff Robbins, Andrew Frock, and Photo below: Kara Small (remote).
