
HJF, Montgomery County, Maryland and BioBuzz are teaming up to bring the research community a new and exciting opportunity
The HJF Networking and Educational Series. Each quarter, a new topic will be presented in HJF's state-of-the-art Conference Facility located at HJF Headquarters, followed by complimentary food and drinks.
 | |
| Date: September 9, 2024 Time: 4-7 p.m. Location: HJF Conference Facility 6720A Rockledge Drive Bethesda, MD 20817 | Agenda for Monday, September 9 4-5pm: FDA panel discussion 5-5:15pm: Break 5:15-5:45pm: Partner Announcements 5:45-7pm: Networking |
Please plan to arrive between 3:30 – 3:45 p.m. as the Panel discussion will begin promptly at 4:00 p.m. | |
Meet the expert FDA/EMA Panel members: EEEC Corp Group – Dr. Carrie Kuehn, PhD, RAC has 25+ years of experience in research epidemiology, medical devices, regulatory and clinical affairs, and patient-focused policy. In practice, Dr. Kuehn applies her strategic remediation skills, and medical device clinical-regulatory knowledge to address the needs of medical products companies and stakeholders across therapeutic areas. She is particularly skilled in supporting remediation efforts, conducting gap analyses, and reorienting strategic plans. Dr. Kuehn addresses complex issues in the pre and post-market space to enhance compliance, hone business practice, and improve patient lives globally. An accomplished researcher, Dr. Kuehn has published in the areas of epidemiology, regulatory affairs, and patient engagement policy. Dr. Kuehn is faculty in the RAQA Graduate Program at Temple University and in the Regulatory Affairs Graduate Program at the Hong Kong University of Science and Technology. FDA Map (www.fdamap.com) - Dr. Mukesh Kumar, PhD, RAC holds a PhD in Biochemistry and is a certified regulatory affair professional by the Regulatory Affairs Professional Society, USA. He consults on regulatory affairs and quality assurance and has created and led numerous teams in the development of pharmaceutical, biotech and medical device products from early-stage development to commercial markets for manufacturers and developers of pharma and biotech, has been involved in more than 100 multi-national clinical trials, has made hundreds of submissions to the US FDA, EMA, and regulators in more than 40 countries. He has conducted GCP, GLP, GMP and GACP audits in the US and countries in Europe, South America and Asia. He has conducted hundreds of training workshops and has authored numerous impactful peer-reviewed articles on healthcare regulations in addition to a popular weekly newsletter on FDA issues with 1000,000+ readers worldwide. He is a frequent consultant to investment companies, government policy think-tanks, social media enterprises, and international business corporations. He is a well-known expert in global regulatory affairs and has been a speaker at several professional and academic organizations worldwide. He is a visiting professor at George Washington University, Washington DC, and Montgomery College, Maryland. Dr Kumar. QuRA Solutions, LLC - Jaspreet Seth, PhD is a Quality and Regulatory Affairs expert with over 20 years of experience in the Life Sciences and Medical Devices sectors, including more than a decade in Operations and Quality management. She has successfully led ISO 13485 and cGMP compliance initiatives for In Vitro Diagnostics (IVDs), Research Use Only products (RUOs), and Laboratory Developed Tests (LDTs), including breakthrough devices and 510k submissions. With a proven track record at industry giants like QIAGEN and smaller molecular diagnostic firms like Molecular Designs, as well as HJF, Jaspreet excels in navigating complex regulations such as 45CFR46, 32CFR219, 21CFR820, 21CFR 210/211 and various ISO, ICH, and USP standards. She is adept at resolving FDA 483s through Root Cause Analysis, CAPAs, and meticulous updates to SOPs. As a subject matter expert in Quality Systems Regulations, Clinical Research Compliance, and CAP accreditation, Jaspreet brings a dynamic and analytical mindset to every project. Her extensive experience in hosting inspections, managing ISO certifications, and implementing GCLP and CAP requirements ensures standards exceed expectations. Salamandra - Tenecia Sullivan, RAC US is the Director for Regulatory Affairs and Clinical Development. She has 20 years of experience in the preparation, management, and submission of over 30 investigational (IND/CTA) and marketing (NDA/MAA) applications (and hundreds of amendments) across multiple therapeutic areas, with an emphasis on providing guidance related to global development programs and clinical trial support, including protocols, study reports, and other study-related documents. In addition to serving as the primary client contact, she also contributes to regulatory submission; provides guidance on regulatory strategy; serves as the primary regulatory contact with agencies; and has supported more than 20 FDA, EMA, Health Canada, and/or MHRA meetings. She also provides guidance to clients on submitting requests for various designations, including breakthrough therapy, fast track, and orphan drugs, and collaborates with her team on cross-functional deliverables, such as gap analyses and due diligence activities. | |
Registration is now closed.
Follow us on LinkedIn for announcements about future events.
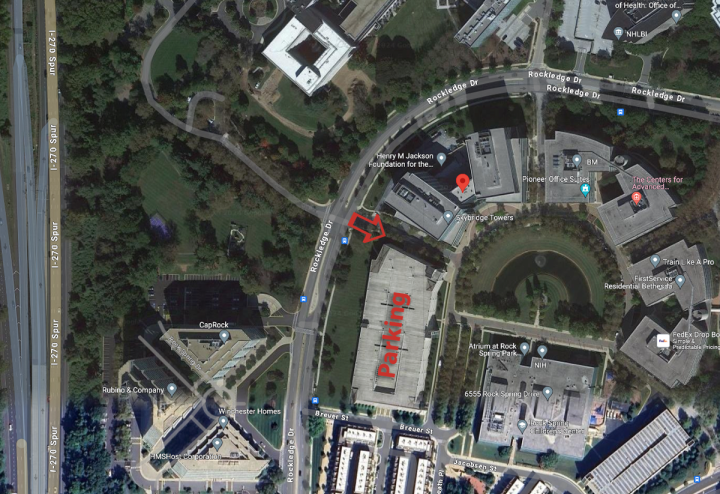
Parking & Directions
Enter through the daily parking gate, the far right entrance.
Parking validation stickers will be available at the event.
Questions? Send an Email to: Linda Yaswen-Corkery